(通讯员 刘静霞)近日,我校必威BETWAY官网鱼类逆境发育遗传学团队在医学外周血管病领域期刊Angiogenesis在线发表了题为“Copper stress impairs angiogenesis and lymphangiogenesis during zebrafish embryogenesis by down-regulating pERK1/2-foxm1-MMP2/9 axis and epigenetically regulating ccbe1 expression”的研究论文,该论文揭示了铜过载损伤斑马鱼胚胎发育过程中血管生成和淋巴管生成的机制。
铜(Cu)在人体内是仅次于锌和铁的第三大微量元素,在机体内发挥一系列重要的生理功能,铜的缺乏与过载都会导致一系列发育缺陷以及疾病的发生。作为脊椎动物循环系统的重要组成部分,血管和淋巴管在各组织、器官之间的气体、液体、营养物质的交换和细胞的运输中发挥必不可少的作用。然而,迄今为止,铜过载对胚胎发育过程中血管和淋巴管的影响及其机制鲜有报道。
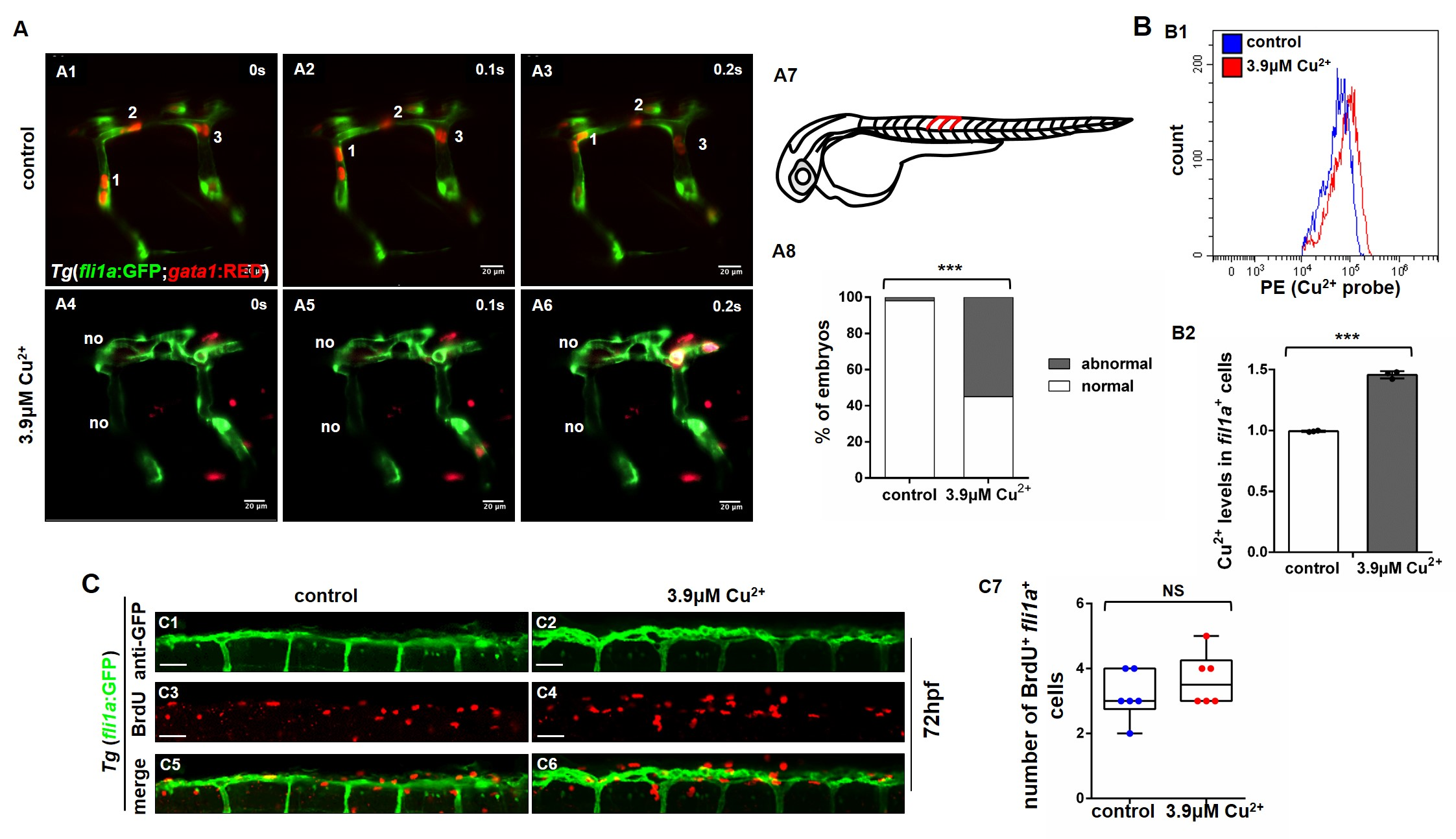
该团队以模式生物斑马鱼与人脐静脉内皮细胞HUVECs为研究对象,发现铜过载的胚胎存在血管生成缺陷以及淋巴管生成缺陷,进一步研究表明铜过载通过下调pERK1/2-foxm1-MMP2/9调控轴,进而影响内皮细胞的迁移而非影响增殖导致血管生成缺陷。此外,铜过载通过上调ccbe1启动子区域的转录因子结合位点的DNA甲基化水平,使转录因子E2F7/8与ccbe1启动子的结合丰度降低,进而下调ccbe1的表达导致淋巴管生成缺陷。
必威BETWAY官网博士研究生邰志鹏和李玲雅为该论文共同第一作者,必威BETWAY官网刘静霞教授为该论文的通讯作者。该研究得到了"蓝色粮仓科技创新"重点专项(2018YFD0900101),“国家自然科学基金”(32070807)等项目的资助。
该学科组一直围绕Cu稳态代谢失衡下的鱼类发育遗传学开展研究。本论文是继该学科组研究成果The FASEB Journal(Jin et al., 2021),BBA-GRM (Zhang et al., 2020),Cell Communication Signaling(Zhao et al., 2020),Frontiers in Immunology(Chen et al., 2020)后的又一重要发现。
【英文摘要】
Molecular transport and cell circulation between tissues and organs through blood and lymphatic vessels are essential for physiological homeostasis in vertebrates. Despite the report of its association with vessel formation in solid tumors, the biological effects of Copper (Cu) accumulation on angiogenesis and lymphangiogenesis during embryogenesis are still unknown. In this study, we unveiled that intersegmental blood circulation was partially blocked in Cu2+-stressed zebrafish embryos and cell migration and tube formation were impaired in Cu2+-stressed mammalian HUVECs. Specifically, Cu2+-stressed embryos showed down-regulation in the expression of amotl2 and its downstream pERK1/2-foxm1-MMP2/9 regulatory axis, and knockdown/knockout of foxm1 in zebrafish embryos phenocopied angiogenesis defects, while FOXM1 knockdown HUVECs phenocopied cell migration and tube formation defects, indicating that excessive Cu2+-induced angiogenesis defects and blocked cell migration via down-regulating amotl2-pERK1/2-foxm1-MMP2/9 regulatory axis in both embryos and mammalian cells. Additionally, thoracic duct was revealed to be partially absent in Cu2+-stressed zebrafish embryos. Specifically, Cu2+-stressed embryos showed down-regulation in the expression of ccbe1 (a gene with pivotal function in lymphangiogenesis) due to the hypermethylation of the E2F7/8 binding sites on ccbe1 promoter to reduce their binding enrichment on the promoter, contributing to the potential mechanisms for down-regulation of ccbe1 and the formation of lymphangiogenesis defects in Cu2+-stressed embryos and mammalian cells. These integrated data demonstrate that Cu2+ stress impairs angiogenesis and lymphangiogenesis via down-regulation of pERK1/2-foxm1-MMP2/9 axis and epigenetic regulation of E2F7/8 transcriptional activity on ccbe1 expression, respectively.
论文链接:https://doi.org/10.1007/s10456-021-09827-0
审核人:刘静霞